Sustained Muscle Contraction Due to Continual Nerve Stimulation of a Neuromuscular Junctuon is
Neuromuscular Junctions
Skeletal muscle cell contraction occurs after a release of calcium ions from internal stores, which is initiated by a neural signal. Each skeletal muscle fiber is controlled by a motor neuron, which conducts signals from the brain or spinal cord to the muscle.
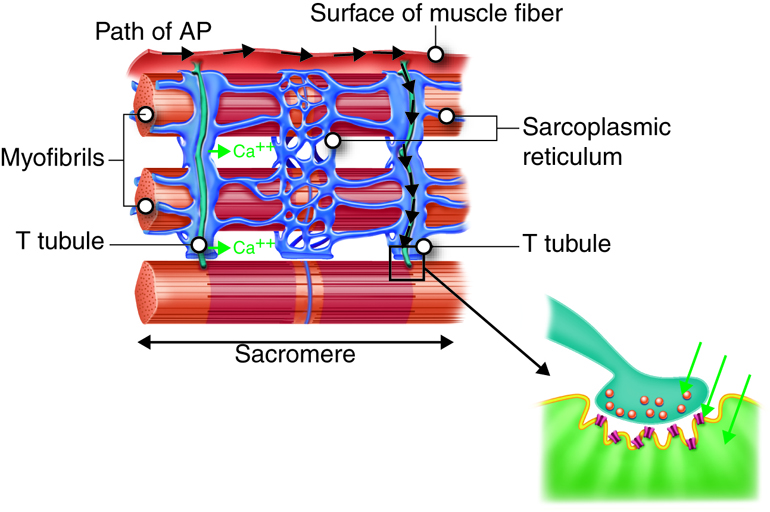 The following list presents an overview of the sequence of events involved in the contraction cycle of skeletal muscle:
The following list presents an overview of the sequence of events involved in the contraction cycle of skeletal muscle:
- The action potential travels down the neuron to the presynaptic axon terminal.
- Voltage-dependent calcium channels open and Ca2+ ions flow from the extracellular fluid into the presynaptic neuron's cytosol.
- The influx of Ca2+ causes neurotransmitter (acetylcholine)-containing vesicles to dock and fuse to the presynaptic neuron's cell membrane.
- Vesicle membrane fusion with the nerve cell membrane results in the emptying of the neurotransmitter into the synaptic cleft; this process is called exocytosis.
- Acetylcholine diffuses into the synaptic cleft and binds to the nicotinic acetylcholine receptors in the motor end-plate.
- The nicotinic acetylcholine receptors are ligand-gated cation channels, and open when bound to acetylcholine.
- The receptors open, allowing sodium ions to flow into the muscle's cytosol.
- The electrochemical gradient across the muscle plasma membrane causes a local depolarization of the motor end-plate.
- The receptors open, allowing sodium ions to flow into and potassium ions to flow out of the muscle's cytosol.
- The electrochemical gradient across the muscle plasma membrane (more sodium moves in than potassium out) causes a local depolarization of the motor end-plate.
- This depolarization initiates an action potential on the muscle fiber cell membrane (sarcolemma) that travels across the surface of the muscle fiber.
- The action potentials travel from the surface of the muscle cell along the membrane of T tubules that penetrate into the cytosol of the cell.
- Action potentials along the T tubules cause voltage-dependent calcium release channels in the sarcoplasmic reticulum to open, and release Ca2+ ions from their storage place in the cisternae.
- Ca2+ ions diffuse through the cytoplasm where they bind to troponin, ultimately allowing myosin to interact with actin in the sarcomere; this sequence of events is called excitation-contraction coupling.
- As long as ATP and some other nutrients are available, the mechanical events of contraction occur.
- Meanwhile, back at the neuromuscular junction, acetylcholine has moved off of the acetylcholine receptor and is degraded by the enzyme acetylcholinesterase (into choline and acetate groups), causing termination of the signal.
- The choline is recycled back into the presynaptic terminal, where it is used to synthesize new acetylcholine molecules.
Anatomy and Physiology of the Neuromuscular Junction
Anatomy
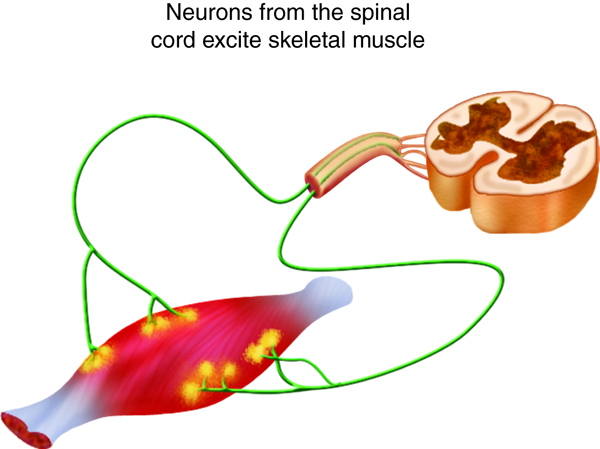
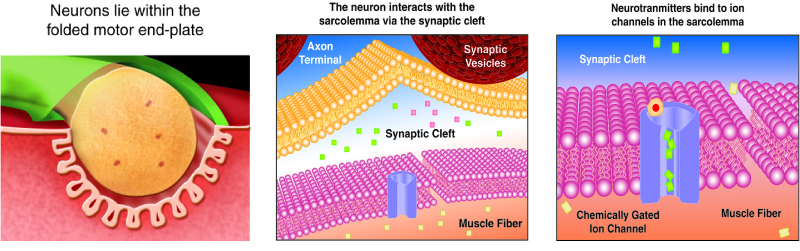
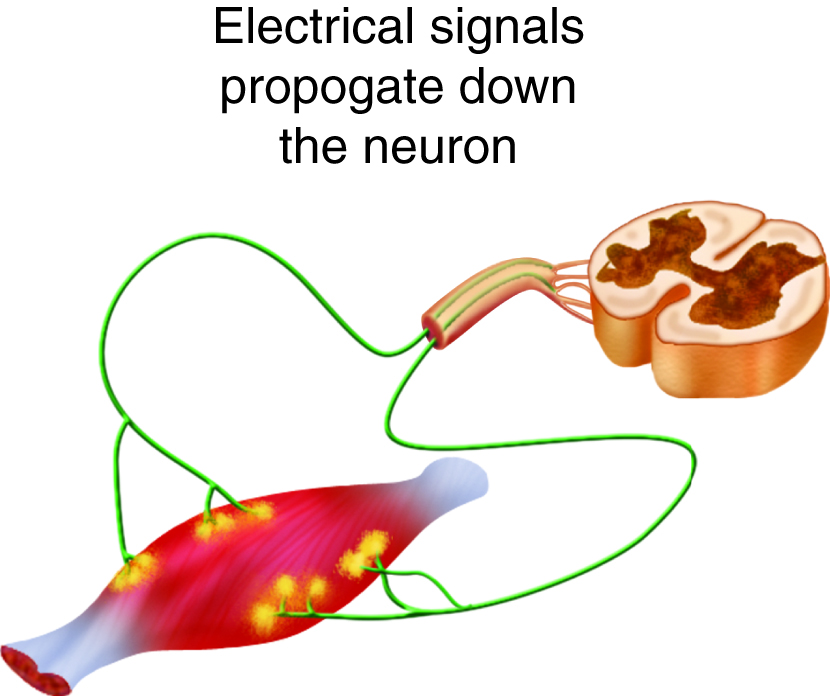
 We stimulate skeletal muscle contraction voluntarily. Electrical signals from the brain through the spinal cord travel through the axon of the motor neuron. The axon then branches through the muscle and connects to the individual muscle fibers at the neuromuscular junction. The folded sarcolemma of the muscle fiber that interacts with the neuron is called the motor end-plate; the folded sarcolemma increases surface area contact with receptors. The ends of the branches of the axon are called the synaptic terminals, and do not actually contact the motor end-plate. A synaptic cleft separates the synaptic terminal from the motor end-plate, but only by a few nanometers.
We stimulate skeletal muscle contraction voluntarily. Electrical signals from the brain through the spinal cord travel through the axon of the motor neuron. The axon then branches through the muscle and connects to the individual muscle fibers at the neuromuscular junction. The folded sarcolemma of the muscle fiber that interacts with the neuron is called the motor end-plate; the folded sarcolemma increases surface area contact with receptors. The ends of the branches of the axon are called the synaptic terminals, and do not actually contact the motor end-plate. A synaptic cleft separates the synaptic terminal from the motor end-plate, but only by a few nanometers.
Communication occurs between a neuron and a muscle fiber through neurotransmitters. Neural excitation causes the release of neurotransmitters from the synaptic terminal into the synaptic cleft, where they can then bind to the appropriate receptors on the motor end-plate. The motor end-plate has folds in the sarcolemma, called junctional folds, that create a large surface area for the neurotransmitter to bind to receptors. Generally, there are many folds and invaginations that increase surface area including junctional folds at the motor endplate and the T-tubules throughout the cells.
Physiology
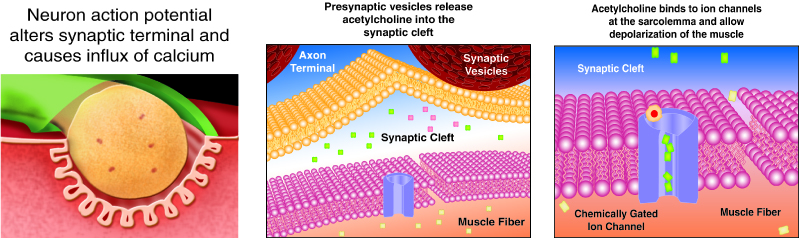
 The neurotransmitter acetylcholine is released when an action potential travels down the axon of the motor neuron, resulting in altered permeability of the synaptic terminal and an influx of calcium into the neuron. The calcium influx triggers synaptic vesicles, which package neurotransmitters, to bind to the presynaptic membrane and to release acetylcholine into the synaptic cleft by exocytosis.
The neurotransmitter acetylcholine is released when an action potential travels down the axon of the motor neuron, resulting in altered permeability of the synaptic terminal and an influx of calcium into the neuron. The calcium influx triggers synaptic vesicles, which package neurotransmitters, to bind to the presynaptic membrane and to release acetylcholine into the synaptic cleft by exocytosis.
Review the section of this course about membranes if you need a refresher.
The balance of ions inside and outside a resting membrane creates an electric potential difference across the membrane. This means that the inside of the sarcolemma has an overall negative charge relative to the outside of the membrane, which has an overall positive charge, causing the membrane to be polarized. Once released from the synaptic terminal, acetylcholine diffuses across the synaptic cleft to the motor end-plate, where it binds to acetylcholine receptors, primarily the nicotinic acetylcholine receptors. This binding causes activation of ion channels in the motor end-plate, which increases permeability of ions via activation of ion channels: sodium ions flow into the muscle and potassium ions flow out. Both sodium and potassium ions contribute to the voltage difference while ion channels control their movement into and out of the cell. As a neurotransmitter binds, these ion channels open, and Na+ ions enter the membrane. This reduces the voltage difference between the inside and outside of the cell, which is called depolarization. As acetylcholine binds at the motor-end plate, this depolarization is called an end-plate potential. It then spreads along the sarcolemma, creating an action potential as voltage-dependent (voltage-gated) sodium channels adjacent to the initial depolarization site open. The action potential moves across the entire cell membrane, creating a wave of depolarization.
After depolarization, the membrane needs to be returned to its resting state. This is called repolarization, during which sodium channels close and potassium channels open. Because positive potassium ions (K+) move from the intracellular space to the extracellular space, this allows the inside of the cell to again become negatively charged relative to the outside. During repolarization, and for some time after, the cell enters a refractory period, during which the membrane cannot become depolarized again. This is because in order to have another action potential, sodium channels need to return to their resting state, which requires an intermediate step with a delay.
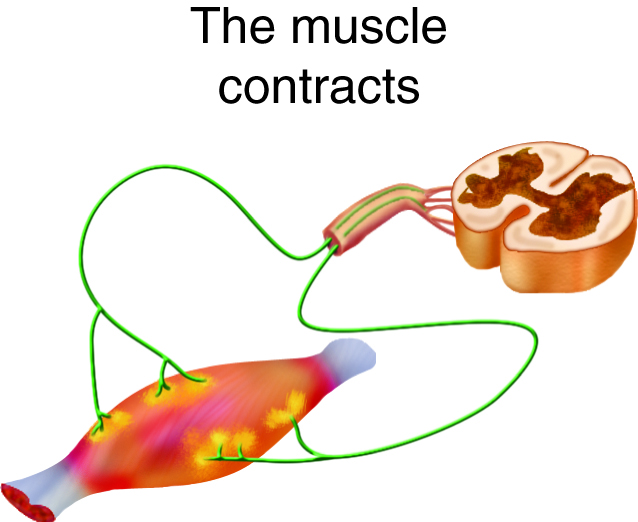
Propagation of an action potential and depolarization of the sarcolemma comprise the excitation portion of excitation-contraction coupling, the connection of electrical activity and mechanical contraction. The structures responsible for coupling this excitation to contraction are the T tubules and sarcoplasmic reticulum (SR). The T tubules are extensions of the sarcolemma and thus carry the action potential along their surface, conducting the wave of depolarization into the interior of the cell. T tubules form triads with the ends of two SR called terminal cisternae. SRs, and especially terminal cisternae, contain high concentrations of Ca2+ ions inside. As an action potential travels along the T tubule, the nearby terminal cisternae open their voltage-dependent calcium release channels, allowing Ca2+ to diffuse into the sarcoplasm. The influx of Ca2+ increases the amount of calcium available to bind to troponin. Troponin bound to Ca2+ undergoes a conformational change that results in tropomyosin moving on the actin filament. When tropomyosin moves, the myosin binding site on the actin is uncovered. This continues as long as excess Ca2+ is available in the sarcoplasm. When there is no more free Ca2+ available to bind to troponin, the contraction will stop. To restore Ca2+ levels back to a resting state, the excess Ca2+ is actively transported back into the SR. In a resting state, Ca2+ is retained inside the SR, keeping sarcoplasmic Ca2+ levels low. Low sarcoplasmic calcium levels prevent unwanted muscle contraction.
Neurotransmitters
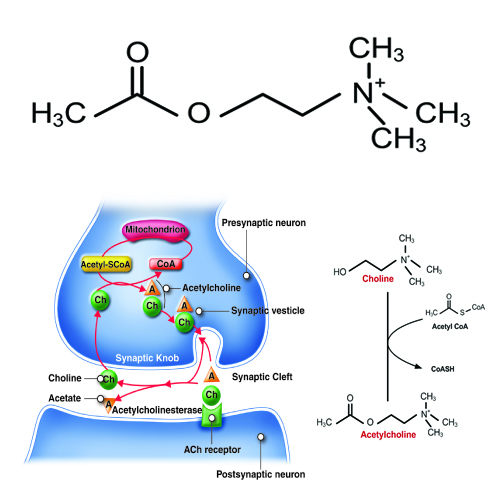 Acetylcholine, often abbreviated as ACh, is a neurotransmitter released by motor neurons that binds to receptors in the motor end-plate. It is an extremely important small molecule in human physiology. On the neuron side of the synaptic cleft, there are typically 300,000 vesicles waiting to be exocytosed at any time and each vesicle contains up to 10,000 molecules of acetylcholine.
Acetylcholine, often abbreviated as ACh, is a neurotransmitter released by motor neurons that binds to receptors in the motor end-plate. It is an extremely important small molecule in human physiology. On the neuron side of the synaptic cleft, there are typically 300,000 vesicles waiting to be exocytosed at any time and each vesicle contains up to 10,000 molecules of acetylcholine.
ACh is produced by the reaction of Acetyl coenzyme A (CoA) with a choline molecule in the neuron cell body. After it is packaged, transported, and released, it binds to the acetylcholine receptor on the motor end-plate; it is degraded in the synaptic cleft by the enzyme acetylcholinesterase (AChE) into acetate (and acetic acid) and choline. The choline is recycled back into the neuron. AChE resides in the synaptic cleft, breaking down ACh so that it does not remain bound to ACh receptors, which would interrupt normal control of muscle contraction. In some cases, insufficient amounts of ACh prevent normal muscle contraction and cause muscle weakness.
Botulinum toxin prevents ACh from being released into the synaptic cleft. With no ACh binding to its receptors at the motor end-plate, no action potential is produced, and muscle contraction cannot occur. Botulinum toxin is produced by Clostridium botulinum, a bacterium sometimes found in improperly canned foods. Ingestion of very small amounts can cause botulism, which can cause death due to the paralysis of skeletal muscles, including those required for breathing.
Cellular Muscle Contraction
ATP supplies the energy for muscle contraction to take place. In addition to its direct role in the cross-bridge cycle, ATP also provides the energy for the active-transport Na+/K+ and Ca2+ pumps. Muscle contraction does not occur without sufficient amounts of ATP. The amount of ATP stored in muscle is very low, only sufficient to power a few seconds worth of contractions. As it is broken down, ATP must therefore be regenerated and replaced quickly to allow for sustained contraction.
One ATP moves one myosin head one step. This can generate three picoNewtons (pN) of isometric force, or move 11 nanometers. Three pN is a very small force—a human bite, generated by muscle, can generate 500 trillion pN of force. And 11 nm is a very small distance— one inch has 25 million nanometers.
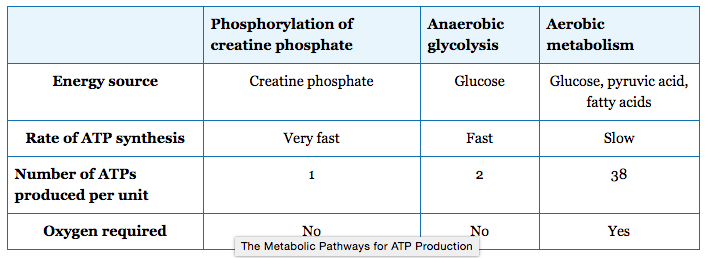
There are three mechanisms by which ATP can be regenerated: creatine phosphate metabolism, anaerobic glycolysis, and aerobic respiration.
Creatine phosphate is a phosphagen, which is a compound that can store energy in its phosphate bonds. In a resting muscle, excess ATP (adenosine triphosphate) transfers its energy to creatine, producing ADP (adenosine diphosphate) and creatine phosphate. When the muscle starts to contract and needs energy, creatine phosphate and ADP are converted into ATP and creatine by the enzyme creatine kinase. This reaction occurs very quickly; thus, phosphagen-derived ATP powers the first few seconds of muscle contraction. However, creatine phosphate can only provide approximately 15 seconds worth of energy, at which point another energy source has to be available.
After the available ATP from creatine phosphate is depleted, muscles generate ATP using glycolysis. Glycolysis is an anaerobic process that breaks down glucose (sugar) to produce ATP; however, glycolysis cannot generate ATP as quickly as creatine phosphate. The sugar used in glycolysis can be provided by blood glucose or by metabolizing glycogen that is stored in the muscle. Each glucose molecule produces two ATP and two molecules of pyruvate, which can be used in aerobic respiration or converted to lactic acid.
If oxygen is available, pyruvic acid is used in aerobic respiration. However, if oxygen is not available, pyruvic acid is converted into lactic acid, which may contribute to muscle fatigue and pain. This occurs during strenuous exercise when high amounts of energy are needed but oxygen cannot be delivered to muscle at a rate fast enough to meet the whole need. Anaerobic glycolysis cannot be sustained for very long (approximately one minute of muscle activity), but it is useful in facilitating short bursts of high-intensity output. Glycolysis does not utilize glucose very efficiently, producing only two ATP molecules per molecule of glucose, and the by-product lactic acid contributes to muscle fatigue as it accumulates. Lactic acid is transported out of the muscle into the bloodstream, but if this does not happen quickly enough, lactic acid can cause cellular pH levels to drop, affecting enzyme activity and interfering with muscle contraction.
Aerobic respiration is the breakdown of glucose in the presence of oxygen to produce carbon dioxide, water, and ATP. Aerobic respiration in the mitochondria of muscles uses glycogen from muscle stores, blood glucose, pyruvic acid, and fatty acids. Approximately 95 percent of the ATP required for resting or moderately active muscles is provided by aerobic respiration. Aerobic respiration is much more efficient than anaerobic glycolysis, producing approximately 38 ATP molecules per molecule of glucose. However, aerobic respiration does not synthesize ATP as quickly as anaerobic glycolysis, meaning that the power output of muscles declines, but lower-power contractions can be sustained for longer periods.
 Muscles require a large amount of energy, and thus require a constant supply of oxygen and nutrients. Blood vessels enter muscle at its surface, after which they are distributed through the entire muscle. Blood vessels and capillaries are found in the connective tissue that surrounds muscle fascicles and fibers, allowing oxygen and nutrients to be supplied to muscle cells and metabolic waste to be removed. Myoglobin, which binds oxygen similarly to hemoglobin and gives muscle its red color, is found in the sarcoplasm.This combination of different energy sources is important for different types of muscle activity. As an analogy, a cup of coffee with lots of sugar provides a quick burst of energy but not for very long. A balanced meal with complex carbohydrates, protein and fats takes longer to impact us, but provides sustained energy.
Muscles require a large amount of energy, and thus require a constant supply of oxygen and nutrients. Blood vessels enter muscle at its surface, after which they are distributed through the entire muscle. Blood vessels and capillaries are found in the connective tissue that surrounds muscle fascicles and fibers, allowing oxygen and nutrients to be supplied to muscle cells and metabolic waste to be removed. Myoglobin, which binds oxygen similarly to hemoglobin and gives muscle its red color, is found in the sarcoplasm.This combination of different energy sources is important for different types of muscle activity. As an analogy, a cup of coffee with lots of sugar provides a quick burst of energy but not for very long. A balanced meal with complex carbohydrates, protein and fats takes longer to impact us, but provides sustained energy.
After the first few seconds of exercise, available ATP is used up. After the next few minutes, cellular glucose and glycogen are depleted. After the next 30 minutes, the body's supply of glucose and glycogen are depleted. After that time, fatty acids and other energy sources are used to make ATP. That's why we should exercise for more than 30 minutes to lose weight (i.e. lose fat). Sometimes, time is important.
Sarcomere Contraction
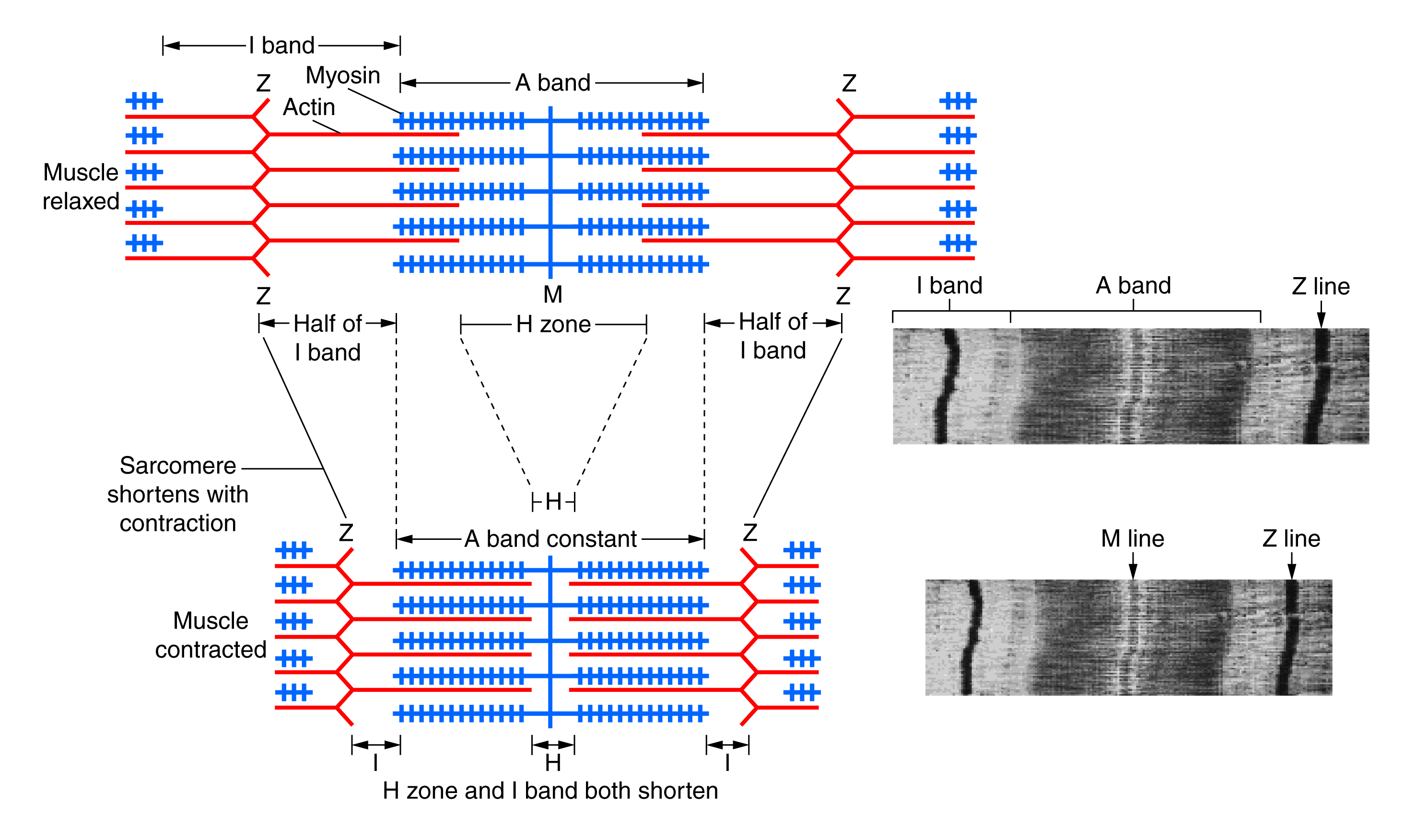
You have already learned about the anatomy of the sarcomere,with its coordinated actin thin filaments and myosin thick filaments. For a muscle cell to contract, the sarcomere must shorten in response to a nerve impulse. The thick and thin filaments do not shorten, but they slide by one another, causing the sarcomere to shorten while the filaments remain the same length. This process is known as the sliding filament model of muscle contraction. The mechanism of contraction is accomplished by the binding of myosin to actin, resulting in the formation of cross-bridges that generate filament movement.
When a sarcomere shortens, some regions shorten while others remain the same length. A sarcomere is defined as the distance between two consecutive Z discs or Z lines. When a muscle contracts, the distance between the Z discs is reduced. The H zone, the central region of the A zone, contains only thick filaments and shortens during contraction. The I band contains only thin filaments and also shortens. The A band does not shorten; it remains the same length, but A bands of adjacent sarcomeres move closer together during contraction. Thin filaments are pulled by the thick filaments towards the center of the sarcomere until the Z discs approach the thick filaments. The zone of overlap, where thin filaments and thick filaments occupy the same area, increases as the thin filaments move inward.
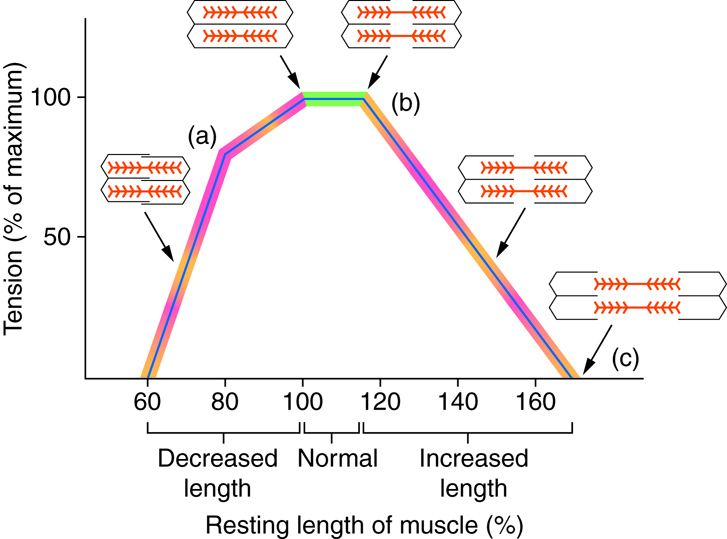
 The ideal length of a sarcomere to produce maximal tension occurs when all of the thick and thin filaments overlap. If a sarcomere is stretched past this ideal length, some of the myosin heads in the thick filaments are not in contact with the actin in the thin filaments, and fewer cross-bridges can form. This results in fewer myosin heads pulling on actin, and less tension is produced. If a sarcomere is shortened, the zone of overlap is reduced as the thin filaments reach the H zone, which is composed of myosin tails. Because myosin heads form cross-bridges, actin will not bind to myosin in this zone, again reducing the tension produced by the muscle. If further shortening of the sarcomere occurs, thin filaments begin to overlap with each other, further reducing cross-bridge formation and the amount of tension produced. If the muscle were stretched to the point where thick and thin filaments do not overlap at all, no cross-bridges are formed, and no tension is produced. This amount of stretching does not usually occur, as accessory proteins and connective tissue oppose extreme stretching.
The ideal length of a sarcomere to produce maximal tension occurs when all of the thick and thin filaments overlap. If a sarcomere is stretched past this ideal length, some of the myosin heads in the thick filaments are not in contact with the actin in the thin filaments, and fewer cross-bridges can form. This results in fewer myosin heads pulling on actin, and less tension is produced. If a sarcomere is shortened, the zone of overlap is reduced as the thin filaments reach the H zone, which is composed of myosin tails. Because myosin heads form cross-bridges, actin will not bind to myosin in this zone, again reducing the tension produced by the muscle. If further shortening of the sarcomere occurs, thin filaments begin to overlap with each other, further reducing cross-bridge formation and the amount of tension produced. If the muscle were stretched to the point where thick and thin filaments do not overlap at all, no cross-bridges are formed, and no tension is produced. This amount of stretching does not usually occur, as accessory proteins and connective tissue oppose extreme stretching.
 With large numbers of relatively weak molecular motors, we can more easily adjust the force to meet our needs. Otherwise, we would regularly be producing too little or too much force for most of our tasks. Also, molecules are only capable of generating small forces based on their molecular structure.
With large numbers of relatively weak molecular motors, we can more easily adjust the force to meet our needs. Otherwise, we would regularly be producing too little or too much force for most of our tasks. Also, molecules are only capable of generating small forces based on their molecular structure.
Neural Stimulation of Contraction
You have already learned about how the information from a neuron ultimately leads to a muscle cell contraction.
Revisit previous material for a review of neuromuscular junctions.
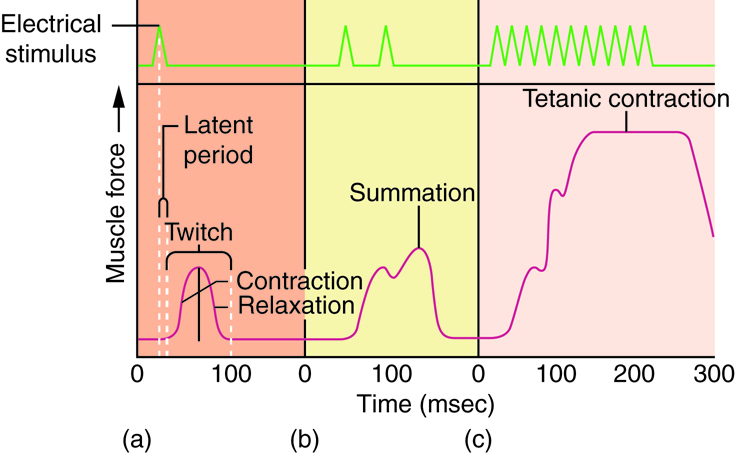 One action potential in a motor neuron produces one contraction. This contraction is called a twitch. We think of "muscle twitches" as spasms that we can't control, but in physiology, a twitch is a technical term describing a muscle response to stimulation. A single twitch does not produce any significant muscle contraction. Multiple action potentials (repeated stimulation) are needed to produce a muscle contraction that can produce work.
One action potential in a motor neuron produces one contraction. This contraction is called a twitch. We think of "muscle twitches" as spasms that we can't control, but in physiology, a twitch is a technical term describing a muscle response to stimulation. A single twitch does not produce any significant muscle contraction. Multiple action potentials (repeated stimulation) are needed to produce a muscle contraction that can produce work.
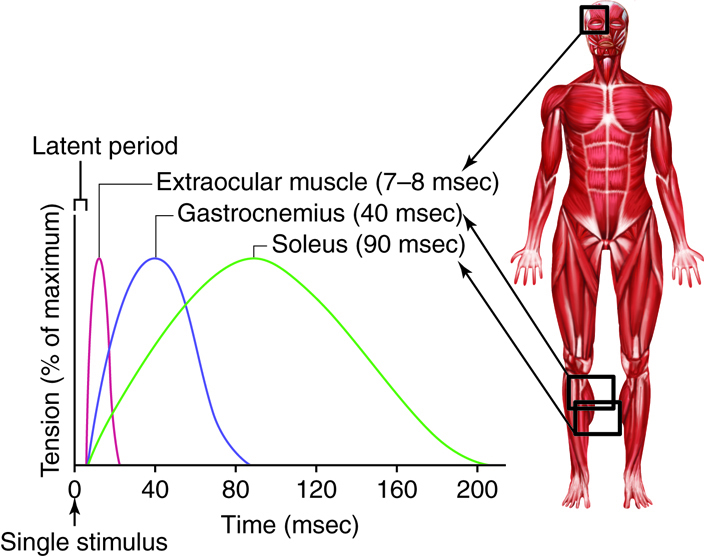
A twitch can last from a few milliseconds up to 100 milliseconds, depending on the muscle type. The tension produced by a single twitch can be measured by a myogram, which produces a graph illustrating the amount of tension produced over time. When combined with a plot of electrical signaling, the myogram shows three phases that each twitch undergoes. The first period is the latent period, during which the action potential is being propagated along the membrane and Ca2+ ions are released from the sarcoplasmic reticulum (SR). No tension or contraction is produced at this point, but the conditions for contraction are being established. This is the phase during which excitation and contraction are being coupled but contraction has yet to occur. The contraction phase occurs after the latent period when calcium is being used to trigger cross-bridge formation. This period lasts from the beginning of contraction to the point of peak tension. The last phase is the relaxation phase, when tension decreases as contraction stops. Calcium is pumped out of the sarcoplasm, back into the SR, and cross-bridge cycling stops. The muscle returns to a resting state. There is a very short refractory period after the relaxation phase (Review the previous material about the physiology of a neuromuscular junction)
A single twitch does not produce any significant muscle activity in a living body. Normal muscle contraction is more sustained, and it can be modified to produce varying amounts of force. This is called a graded muscle response. The tension produced in a skeletal muscle is a function of both the frequency of neural stimulation and the number of motor neurons involved.
The rate at which a motor neuron delivers action potentials affects the contraction produced in a muscle cell. If a muscle cell is stimulated while a previous twitch is still occurring, the second twitch will not have the same strength as the first; it will be stronger. This effect is called summation, or wave summation, because the effects of successive neural stimuli are summed, or added together. This occurs because the second stimulus releases more Ca2+ ions, which become available while the muscle is still contracting from the first stimulus (the first wave of calcium ions released). This allows for more cross-bridge formation and greater contraction. Because the second stimulus has to arrive before the first twitch has completed, the frequency of stimulus determines whether summation occurs or not.
If the frequency of stimulation increases to the point at which each successive stimulus sums with the force generated from the previous stimulus, muscle tension continues to rise until the tension generated reaches a peak point. The tension at this point is about three to four times higher than the tension of a single twitch; this is referred to as incomplete tetanus. Tetanus is defined as continuous fused contraction. During incomplete tetanus, the muscle goes through quick cycles of contraction with a short relaxation phase. If the stimulus frequency is so high that the relaxation phase disappears completely, contractions become continuous in a process called complete tetanus. This occurs when Ca2+ concentrations in the sarcoplasm reach a point at which contractions can continue uninterrupted. This contraction continues until the muscle fatigues and can no longer produce tension.
This type of tetanus is not the same as the disease of the same name that is distinguished by severe sustained contraction of skeletal muscles. The disease, which can be fatal if left untreated, is caused by the bacterium Clostridium tetani, which is present in most environments. The toxin from the bacterium affects how motor neurons communicate and control muscle contractions, resulting in muscle spasms or sustained contractions, also known as "lockjaw."
Slightly different from incomplete tetanus is the phenomenon of treppe. Treppe (from the German term for step, referring to stepwise increases in contraction) is a condition in which successive stimuli produce a greater amount of tension, even though tension goes back to the resting state between stimuli (in tetanus, tension does not decrease to the resting state between stimuli). Treppe is similar to tetanus in that the first twitch releases calcium into the sarcoplasm, some of which will not be taken back up before the next contraction. Each stimulus afterward releases more calcium, but there is still some calcium present in the sarcoplasm from the previous stimulus. This extra calcium permits more cross-bridge formation and greater contraction with each additional stimulus up to the point where added calcium cannot be utilized. At this point, successive stimuli will produce a uniform amount of tension.
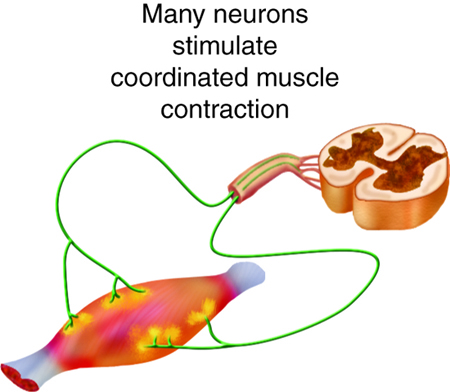
The strength of contractions is controlled not only by the frequency of stimuli but also by the number of motor units involved in a contraction. A motor unit is defined as a single motor neuron and the corresponding muscle fibers it controls. Increasing the frequency of neural stimulation can increase the tension produced by a single motor unit, but this can only produce a limited amount of tension in a skeletal muscle. To produce more tension in an entire skeletal muscle, the number of motor units involved in contraction must be increased. This process is called recruitment.
The size of motor units varies with the sizes of muscle. Small muscles contain smaller motor units and are most useful for fine motor movements. Larger muscles tend to have larger motor units because they are generally not involved in fine control. Even within a muscle, motor units vary in size. Generally, when a muscle contracts, small motor units will be the first ones recruited in a muscle, with larger motor units added as more force is needed.
All of the motor units in a muscle can be active simultaneously, producing a very powerful contraction. This cannot last for very long because of the energy requirements of muscle contraction. To prevent complete muscle fatigue, typically motor units in a given muscle are not all simultaneously active, but instead, some motor units rest, while others are active, allowing for longer muscle contractions by the muscle as a whole.
The action potentials produced by pacemaker cells in cardiac muscle are longer than those produced by motor neurons that stimulate skeletal muscle contraction. Thus, cardiac contractions are approximately ten times longer than skeletal muscle contractions. Because of long refractory periods, new action potential cannot reach a cardiac muscle cell before it has entered the relaxation phase, meaning that the sustained contractions of tetanus are impossible. If tetanus were to occur, the heart would not beat regularly, interrupting the flow of blood through the body.
Skeletal Muscle Tissue and Fiber Types
Muscle contractions are among the largest energy-consuming processes in the body, which is not surprising considering the work that muscles constantly do. Skeletal muscles move the body in obvious ways such as walking and in less noticeable ways such as facilitating respiration. The structure of muscle cells at the microscopic level allows them to convert the chemical energy found in ATP into the mechanical energy of movement. The proteins actin and myosin play large roles in producing this movement.
Skeletal Muscle Anatomy
Recall all of the structures of the fused skeletal muscle cell. If you need to, review organelles and structures specific to the skeletal muscle cells.
Structures analogous to other cell organelles:
- Sarcolemma—the membrane of the fused skeletal fiber.
- Sarcoplasm—the cytoplasm of the fused skeletal fiber.
- Sarcoplasmic reticulum—the endoplasmic reticulum of the fused skeletal fiber.
Specialized structures in muscle cells:
- Transverse tubules (T tubules)—sarcolemma tubes filled with extracellular fluid that coordinate conduction in large muscle cells.
- Terminal cisternae—enlarged sarcoplasmic reticulum structures store calcium and surround T tubules.
- Triad—one T tubule and two terminal cisternae.
Skeletal Muscle Fiber Types
There are three main types of skeletal muscle fibers (cells): slow oxidative (SO), which primarily uses aerobic respiration; fast oxidative (FO), which is an intermediate between slow oxidative and fast glycolytic fibers; and fast glycolytic (FG), which primarily uses anaerobic glycolysis. Fibers are defined as slow or fast based on how quickly they contract. The speed of contraction is dependent on how quickly the ATPase of myosin can hydrolyse ATP to produce cross-bridge action. Fast fibers hydrolyse ATP approximately twice as quickly as slow fibers, resulting in quicker cross-bridge cycling. The primary metabolic pathway used determines whether a fiber is oxidative or glycolytic. If a fiber primarily produces ATP through aerobic pathways, it is oxidative. Glycolytic fibers primarily create ATP through anaerobic glycolysis.
Since SO fibers function for long periods without fatigue, they are used to maintain posture, producing isometric contractions useful for stabilizing bones and joints, and making small movements that happen often but do not require large amounts of energy. They do not produce high tension, so they are not used for powerful, fast movements that require high amounts of energy and rapid cross-bridge cycling.
FO fibers are sometimes called intermediate fibers because they possess characteristics that are intermediate between fast fibers and slow fibers. They produce ATP relatively quickly, more quickly than SO fibers, and thus can produce relatively high amounts of tension. They are oxidative because they produce ATP aerobically, possess high numbers of mitochondria, and do not fatigue quickly. FO fibers do not possess significant myoglobin, giving them a lighter color than the red SO fibers. FO fibers are used primarily for movements, such as walking, that require more energy than postural control but less energy than an explosive movement such as sprinting. FO fibers are useful for this type of movement because they produce more tension than SO fibers and they are more fatigue-resistant than FG fibers.
FG fibers primarily use anaerobic glycolysis as their ATP source. They have a large diameter and possess high amounts of glycogen, which is used in glycolysis to generate ATP quickly; thus, they produce high levels of tension. Because they do not primarily use aerobic metabolism, they do not possess substantial numbers of mitochondria nor large amounts of myoglobin and therefore have a white color. FG fibers are used to produce rapid, forceful contractions to make quick, powerful movements. However, these fibers fatigue quickly, permitting them to only be used for short periods.
Most muscles (organs) possess a mixture of each fiber (cell) type. The predominant fiber type in a muscle is determined by the primary function of the muscle. Large muscles used for powerful movements contain more fast fibers than slow fibers. As such, different muscles have different speeds and different abilities to maintain contraction over time. The proportion of these different kinds of muscle fibers will vary among different people and can change within a person with conditioning.
Licenses and Attributions
Source: https://www.coursehero.com/study-guides/cuny-csi-ap-1/neuromuscular-junctions-and-muscle-contractions/
0 Response to "Sustained Muscle Contraction Due to Continual Nerve Stimulation of a Neuromuscular Junctuon is"
Post a Comment